Abstract
Introduction: A challenge of chimeric antigen receptor (CAR) T-cell therapy is the en-masse activation of tumor-reactive T-cells that can lead to cardiovascular, respiratory, and neurologic toxicities, including cytokine release syndrome (CRS) and neurotoxicity (NT). Recent studies found that elevated levels of cytokines and inflammatory markers pre-treatment are associated with severe toxicities in B cell lymphoma patients receiving axicabtagene ciloleucel. However, less is known about patients with relapsed/refractory multiple myeloma (RRMM) treated with commercial idecabtagene vicleucel (ide-cel). We evaluated the associations of serum cytokines and inflammatory biomarkers with immune-mediated toxicities and progression-free survival (PFS) in RRMM patients treated with commercial ide-cel.
Methods: Serum samples were collected at baseline (within 45 days of lymphodepleting chemotherapy) and daily from infusion until hospital discharge. The Ella automated simple plex immunoassay system (ProteinSimple) was used to measure cytokines including GM-CSF, IL2, IL6, IL15, IFNγ, TNFα, and Angiopoietin 1&2. Standard clinical tests were used to measure ferritin and C-reactive protein at baseline (pre-lymphodepleting chemotherapy), infusion, and daily until hospital discharge. Treatment-related toxicities were graded based on ASTCT guidelines and managed according to institutional policies. Treatment responses were graded based on IMWG response criteria. Chi-square and Kruskal-Wallis tests were used to examine the distribution of each cytokine and inflammatory biomarker at baseline, infusion, and peak (highest level between day one post infusion and last day of hospitalization) by any grade and ≥ grade 2 CRS and NT. For cytokines or inflammatory biomarkers associated with CRS and NT, maximally selected rank statistics were used to determine optimal cut-points for PFS, and Kaplan-Meier survival curves and log-rank tests were used to examine PFS by optimal cut-points of selected cytokines and inflammatory biomarkers.
Results: 28 patients who received ide-cel by May 1st, 2022 were included. Median age was 65 years. 38% of patients had high-risk cytogenetics and 54% had penta-refractory disease. Median number of prior lines of therapy was 6 (range 4-13). CRS was seen in 79% (≥ grade 2: 14%) and NT in 11% (≥ grade 2: 7%) of patients. Tocilizumab and steroids were used in 79% and 32% of patients, respectively. With a median follow up of 6.2 months, best overall response rate was 82%, with 57% of patients achieving a complete response or better. Of those, 69% were minimal residual disease negative. Four patients died of myeloma progression.
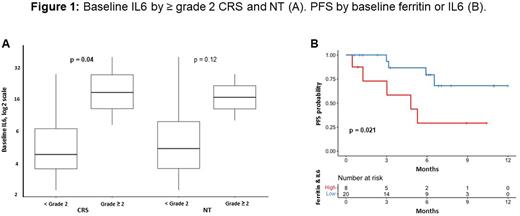
Patients with any grade versus no CRS had higher levels of baseline ferritin (402 vs 133 ng/mL; P=0.045), peak ferritin (2804 vs 191 ng/mL; P<0.001), and peak IL6 (3681 vs 20 pg/mL; P<0.001). Patients with ≥ grade 2 vs < grade 2 CRS had higher levels of baseline IL6 (19 vs 5 pg/mL; P=0.04; Figure 1). A positive but not statistically significant association was observed for baseline IL6 with ≥ grade 2 vs < grade 2 NT (19 vs 6 pg/mL; P=0.12). There were positive correlations between baseline and peak ferritin (r=0.43; P=0.02) and baseline ferritin and IL6 (r=0.58; P=0.005), but no correlation between baseline and peak IL6 (r=-0.06; P=0.8). Using optimal cut-points for baseline ferritin (1700 ng/mL) and IL6 (10 pg/mL), patients with high (n=5) vs low (n=23) baseline ferritin had an inferior PFS (median PFS of 4.8 months vs not reached [NR]; P=0.0005), and patients with high (n=6) vs low (n=16) baseline IL6 had an inferior PFS (median PFS of 3 months vs NR; P=0.069). When combining baseline ferritin and IL6, patients with either high (n=8) vs low (n=20) ferritin or IL6 had an inferior PFS (median PFS of 4.8 months vs NR; P=0.021; Figure 1). No other differences in inflammatory biomarkers or cytokines by toxicities or PFS were identified.
Conclusions: In our single center experience, we found associations between ferritin and IL6 with CRS and NT. Median PFS was inferior in patients with elevated baseline ferritin and IL6. This suggests that a pre-existing pro-inflammatory state puts ide-cel patients at risk for toxicities in addition to inferior PFS. Tailoring toxicity and additional therapeutic management to patient risk may mitigate morbidity and mortality.
Disclosures
Hansen:Survivorship: Honoraria; BMS IMW Ide-Cel Academic Advisory Board: Membership on an entity's Board of Directors or advisory committees; OncLive: Honoraria. Freeman:Janssen: Honoraria, Research Funding; Amgen: Honoraria; Sanofi: Honoraria; Incyte: Honoraria; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Faramand:Kite/Gilead: Research Funding; Novartis: Research Funding. Blue:Jassen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria; Sanofi: Consultancy, Speakers Bureau. Elmariah:Bristol Myers Squibb: Research Funding. Jain:BMS: Consultancy; MyeloidTx: Consultancy; Incyte: Research Funding; Novartis: Consultancy; Kite Pharma: Consultancy, Research Funding. Liu:Sanofi: Speakers Bureau. Shain:Bristol Myers Squibb (BMS), Janssen, GlaxoSmithKline (GSK), Adaptive, Sanofi, and Takeda, and Amgen: Honoraria; GSK, Janssen and BMS: Membership on an entity's Board of Directors or advisory committees; GSK, BMS, Sanofi, Karyopharm, Takeda, Janssen, Adaptive and Amgen: Speakers Bureau; AbbVie and Karyopharm: Research Funding; Janssen and BMS: Other: PI of clinical trials. Locke:CAREducation: Other: Education or editorial activity; Sana: Consultancy; Clinical Care Options Oncology: Other: Education or editorial activity; Takeda: Consultancy; CERo Therapeutics: Research Funding; Daiichi Sankyo: Consultancy; ), National Cancer Institute: Research Funding; Imedex: Other: Education or editorial activity; ASH: Other: Education or editorial activity; Aptitude Health: Other: Education or editorial activity; Leukemia and Lymphoma Society: Research Funding; BioPharm Communications: Other: Education or editorial activity; BMS: Research Funding; Society for Immunotherapy of Cancer: Other: Education or editorial activity; A2: Consultancy; Celgene: Consultancy; Other: Patents & Royalties: patents, royalties, other intellectual property from several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy.; Wugen: Consultancy; Umoja: Consultancy; Novartis: Consultancy, Research Funding; Legend Biotech: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Janssen: Consultancy; Iovance: Consultancy; GammaDelta Therapeutics: Consultancy; Emerging Therapy Solutions Gerson Lehrman Group: Consultancy; EcoR1: Consultancy; Cowen: Consultancy; Calibr: Consultancy; Cellular Biomedicine Group: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Bluebird Bio: Consultancy, Research Funding; Allogene: Consultancy, Research Funding; Amgen: Consultancy. Alsina:BMS, Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal